- Home
- EventsEvents
- Product Launches
- CategoriesCategories
- Advertise
- Opinion

Brewers know: Beer is probably the drink that is most sensitive to oxygen. The so-called oxidation taste occurs when oxygen uptakes are too high. Trend beers with the rediscovered cold hopping, formerly called "hop stoppers", change their taste quite quickly when oxygen is absorbed. But ordinary light beers are also particularly sensitive to oxygen. Darker beers, on the other hand, often show a considerable taste tolerance to oxygen levels beyond 0.1 mg/l.
The oxygen uptake during beer production begins when the malt is crushed and does not end when the beer leaves the brewery, because oxygen penetrates the seals of crown caps, for example – until the beer is consumed. Beer constituents react so quickly with oxygen that dissolved oxygen measurements are necessary immediately after the respective processes such as tank filling, filtration or filling. For example, an oxidized beer with the typical changes in taste often has hardly any measurable oxygen contents, and yet the loss of quality is significant.
The use of additives such as bisulfite or ascorbic acid as oxygen scavenger is common in other countries outside Germany, especially for filling into PET bottles. This is because these containers, as well as the most commonly used closure made of PE or PP, allow oxygen to migrate in relevant orders of magnitude in a short time. However, as an antioxidant for beer, ascorbic acid is a double-edged sword. Oxidation processes – not only in beer, but also in other beverages such as soft drinks or juices – are complex processes with many intermediate stages, and thus much more than the transition from oxygen to a specific recipient molecule. Are the polyphenols in malt or hops beneficial antioxidants or harmful turbidifiers? This is a chapter in itself, which is still being explored by science.
To meet this challenge, breweries are developing different approaches to quality assurance: from low-polyphenol malts to polyphenol-rich cone hopping, or from strategies for preservation to precipitation and reduction of polyphenols using PVPP.
Experts agree that avoiding or reducing oxygen uptake is the most important part of achieving the longest possible taste stability. For this purpose, tanks are prestressed with inert gas, for example, and they are often cleaned under this material so that they do not have to accept any corresponding losses. Is the inert gas CO2, then only acidic purification is possible with preservation of the gas. Will N2 used, so dissolved N2content in the beer in order to achieve undisturbed filling performance and not to produce atypical foam structures in the consumer. Alternatives such as argon would be very expensive.
In principle, tank pressures should be selected as low as they correspond to the CO2partial pressure of the beer and pumps can be used for necessary pressure increases. When it comes to filtration, it is recommended to work with degassed water without fail. Oxygen contents of less than 0.2 mg/l should be sought – as well as for extension water, for example in flash pasteurisation – because non-degassed water has around 10 mg/l.
The oxygen measurement in the testing of ejection processes with mixed phase separation shows a grievance much earlier than, for example, the original wort measurement. Today's requirements and possibilities make it seem sensible to even leave the previously accustomed unit mg/l (~ ppm) for oxygen uptake and switch to the more manageable unit μg/l (~ppb1) to change. It is not uncommon for beers before bottling to have 5 μg/l and below, which is less than 0.005 mg/l. A few years ago, filling systems that achieved total oxygen uptakes of 150 μg/l were common, but now even 20 μg/l are possible with the appropriate technology.
Total oxygen, often referred to as TPO for "total package(d) oxygen", includes both the oxygen dissolved in the beer and that which is in the headspace of a sealed container. The method of determining the "air in the headspace" by transferring this headspace gas into a burette filled with lye for the adsorption of the CO2 is still used today for the sake of simplicity. But the gas composition in the headspace does not only consist of CO2 and air with the usual oxygen content. Thus, the "air in the headspace" is less suitable for determining the TPO. For a precise determination of the total oxygen, measuring devices are now available that can more or less automatically measure both the headspace oxygen and the oxygen dissolved in the beverage. Together with temperature and complex pressure measurements, the headspace volume, the CO2content and even other dissolved gases.
Almost 40 years ago, the brewmasters Uhlig and Vilachá of the Polar Brewery in Caracas, Venezuela, developed a formula to determine the TPO with high precision by means of an oxygen meter for dissolved oxygen and the determination of temperature, headspace and filling volume. For this purpose, the containers must be brought into a gas equilibrium state, which is effected by shaking. This method has the great advantage that all process steps in beer production and bottling can be controlled with one and the same portable device, even gas-in-gas measurements are possible today; for measurements from cylinders or cans, only an additional piercing apparatus with inert gas supply is required. Finally, the dissolved oxygen of a gas-equilibrium beverage in a bottle or can must be multiplied by a calculated factor to obtain the TPO. For conventional containers and filling temperatures, this factor is between 2 and 3. Modern filling machines achieve measured values of dissolved oxygen that are even lower than those measured in front of the filling machine when shaken containers. In comparison, measurements on unshaken containers provide information on the effectiveness of the indispensable foaming before closing or the design and adjustment of the under-lid gassing in a can seamer.
This comparison reveals: In modern filling systems, the filling process itself contributes only 10 to 20 percent of the total oxygen in a filled and sealed container. The oxygen uptake during filling must always be seen in relation to the necessary inert gas consumption. Whereas in the past, even at high consumption, such low oxygen uptakes were not possible and perhaps not considered necessary, significant improvements have now been achieved.
Depending on the necessary or selected filling process, the use of N2 possible – today, this can be produced in-house using molecular sieves at an attractively priced price.
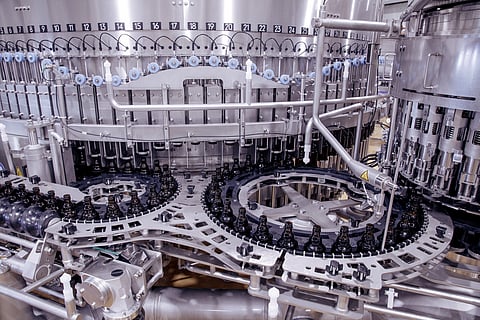
With modern technology, breweries realize reliable quality in the production of their beers with a low oxygen content. Above all, this makes the use of additives such as bisulfite or ascorbic acid as oxygen scavenger superfluous. This is precisely where KHS came in with the development of the modular Innofill Glass DRS ECO filling system. This has long since arrived in practice. Among others, the OeTTINGER brewery in Mönchengladbach relies on the solution. According to the customer, the filler allows low-oxygen filling, which did not previously exist in this form.
This is because the new filling machine allows less oxygen into the beer – and requires less CO instead of more2 than their predecessors. In the filling of bottles, a new type of hollow probe filler makes it possible to2demand on the one hand, even lower oxygen uptake is possible on the other. First, the container is evacuated through the vacuum channel, and then with CO2-Gas purged. A rinsing process patented by KHS is used for this purpose. Subsequently, as usual, the container is prestressed with the inert gas to filling pressure. For example, the extremely low total oxygen uptake of 20 ppb at a CO2-Consumption of 160 g/hl possible. With further reduced CO2consumption to, for example, 110 g/hl, which is half as much as was previously the case, a very low total oxygen uptake of 40 ppb is still achievable. The desired total oxygen uptake can be selected: For example, an automatic type changeover to a particularly oxygen-sensitive cold-hopped beer, a darker beer that is less sensitive to oxygen or even to a completely different filling for lemonades with the greatest possible CO2-Saving can be adjusted.
Many breweries have to buy carbon dioxide. Due to the rising price, this is a growing cost factor. The lower the consumption for the filled bottle, the more efficient the production, and the lower the oxygen uptake, the better the product quality. KHS has perfectly reconciled these two points with the Innofill Glass DRS ECO. In addition, digital and automated systems ensure synchronization of filling processes and thus a significant increase in efficiency.
There are currently 23 Innofill Glass DRS-ECO reference plants worldwide.
1 ppb = parts per billion (German: parts per billion); For example, 1 ppb is equivalent to 1 μg per 1 kg
Click HERE to subscribe to our FREE Weekly Newsletter
